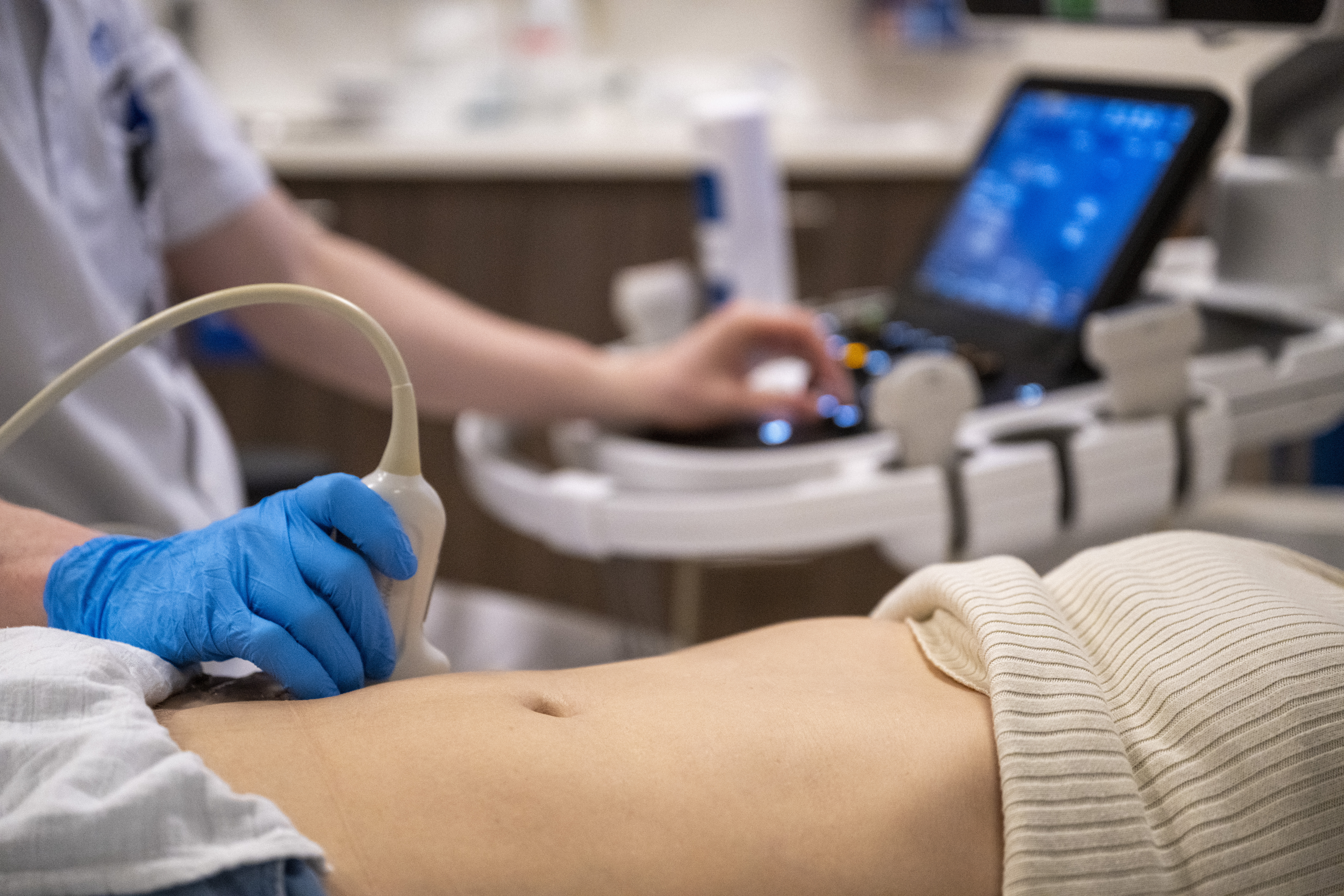
The UK Health Security Agency has alerted manufacturers to a manufacturer recall of Ebrington Medical AquaUltra clear ultrasound gel due to the potential risk of microbial contamination.
An investigation by Public Health Authorities found a risk of Burkholderia stabilis bacteria, which may lead to infection if the affected gel is used on high-risk patients.
NHS Supply Chain has now suspended supply of the non-sterile ultrasound product.
'Essential to be aware of this'
A representative for the SoR said: "It is essential that sonographers are aware of this product recall and adhere to UKHSA guidance to protect patients. They may also be well placed to support other ultrasound users to implement best practice guidance within the wider service."
Risk of infection becomes higher in patients with cystic fibrosis, severe lung disease, severely immunocompromised patients and intensive care patients.
Healthcare providers are advised to cease use and quarantine the affected products, and contact their local NHS Supply Chain Customer Services Advisor with full details of any affected products in their possession.
Alignment to the guidance
Where potential alternatives are identified, customers are advised to consult clinical experts to ensure these products are suitable for their organisation.
UKHSA has published recommendations on guidance for the safe use of ultrasound gel, which outlines measures to reduce the risk to patients associated with the use of non-sterile ultrasound gel and when to use sterile gel. UKHSA previously issued a National Patient Safety Alert to ensure alignment to this guidance, and the contents of this alert remain valid.
More information on the affected product code and potential alternatives, as well as the full instructions in the Field Safety Notice, can be found here.
(Image: Photo by Pancakes Pictures, via GettyImages)
